
 Here is a very simple way to demonstrate that
water contains hydrogen and oxygen. In order to
show electrolysis, you'll need a 9 volt battery,
some bits of wire, tape, a small
saucer, some salt, and two small
pencils sharpened at each end.
Here is a very simple way to demonstrate that
water contains hydrogen and oxygen. In order to
show electrolysis, you'll need a 9 volt battery,
some bits of wire, tape, a small
saucer, some salt, and two small
pencils sharpened at each end.
You can use this demonstration in lower grades
just to illustrate that oxygen and hydrogen are
the components of water. But we've also included
enough information to make it useful for high
school chemistry students.
However you make use of the demo, it only takes
seconds to set up, requires no special equipment,
and it works every time! The first time we saw
this demonstrated we were amazed at how simple
it is to separate water into its components ...
no complicated tubing, glassware, and electrical
power supply needed!
You can use a clear glass dish, and show the process
on an overhead projector.
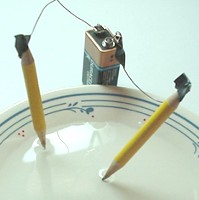  Here's the setup. That's all there is!
Here's the setup. That's all there is!
Add a sprinkle or two of salt to the water
... not much is needed. Each pencil becomes an
electrode, and the moment you hook up the battery,
bubbles will begin appearing at the tip of each
pencil: oxygen at the positive electrode, and
hydrogen at the negative one.
You can try collecting the gases with inverted
small test tubes, and use a burning splint to
test for hydrogen (the tube will pop) and a glowing
splint to test for oxygen (the splint will burst
into flame).
Below on this page we've included some terms describing
electrolysis, and an explanation of the process
that is suitable for high school chemistry students.
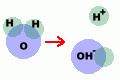 When
you add salt to the water,
the salt ions (which are highly polar) help
pull the water molecules apart into ions too.
Each part of the water molecule has a charge.
The OH- ion is negative,
and the H+ ion is positive. When
you add salt to the water,
the salt ions (which are highly polar) help
pull the water molecules apart into ions too.
Each part of the water molecule has a charge.
The OH- ion is negative,
and the H+ ion is positive.
This solution in water forms an electrolyte,
allowing current
to flow when a voltage is applied. The
H+ ions, called cations,
move toward the cathode (negative electrode),
and the OH- ions, called anions,
move toward the anode (positive electrode).
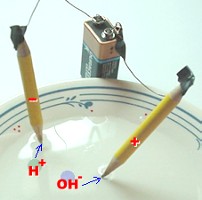 If
you add some universal indicator solution
to the salt water, you will be able to see
a colour change corresponding to acid
near the cathode (H+ ions in water)
and base near the anode (OH-
ions in water). If
you add some universal indicator solution
to the salt water, you will be able to see
a colour change corresponding to acid
near the cathode (H+ ions in water)
and base near the anode (OH-
ions in water).
At the anode, water is oxidized:
2H2O
—> O2 + 4H+
+ 4e-
At the cathode, water is reduced:
4H2O
+ 4e- —> 2H2
+ 4OH-
Note that there is a net balance of electrons
in the water.
Bubbles of oxygen gas (O2) form at
the anode, and bubbles of hydrogen gas (H2)
form at the cathode.
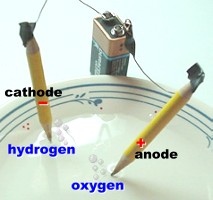

The bubbles are
easily seen. Twice as much hydrogen gas is produced
as oxygen gas.
The net reaction:
2H2O —> 2H2
+ O2
|